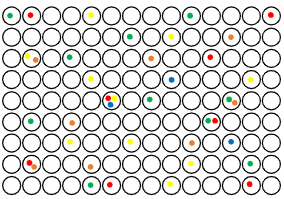
Atila Multiplex Digital PCR Quantification Assay
Absolute quantification of clinically important diseases using digital PCR technology.
- Oncology
- Reproductive Health including NIPT
- Infectious Disease Testing (IDT)
- Absolute Quantification
- Extraction Free
- Simplest dPCR Test for Infectious Diseases
- Fastest dPCR Test, Results Within an Hour
Each kit includes 100 tests.
Atila Multiplex Digital PCR Quantification Assay
Digital PCR (dPCR) is a cutting-edge molecular technology designed for the absolute quantification of nucleic acids with unmatched precision and sensitivity. After partitioning the sample, each reaction undergoes end-point PCR. Partitions are then analyzed for fluorescence to determine whether a target is present (positive) or absent (negative). This binary readout enables absolute quantification of target molecules without the need for standard curves. As a result, digital PCR (dPCR) offers greater precision and sensitivity compared to traditional qPCR. This enables more confident clinical decisions with regards to accurate diagnosis of various infections or abnormalities.
Atila has created a digital platform for analysis of clinically important diseases. Our ready-to-use, simple and extraction-free workflow reduces lab hands on time making this product an ideal choice to scale up DNA quantification projects and it allows for quantification and screening for multiple targets in a single well. The hands on time is less than 20 minutes and multiple patient samples can be analyzed in a single plate for high- throughput testing. Our assay provides superior sensitivity and specificity paving the way for accurate diagnosis of various diseases and conditions.
Features Include
Technical Specs
Workflow Overview for the Atila Multiplex Digital PCR Quantification Assay
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.
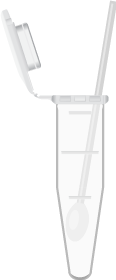
Assay sample type in sample tube

Add up to 750µL 1X Atila sample buffer.
Vortex & incubate at 95°C for 10 minutes.
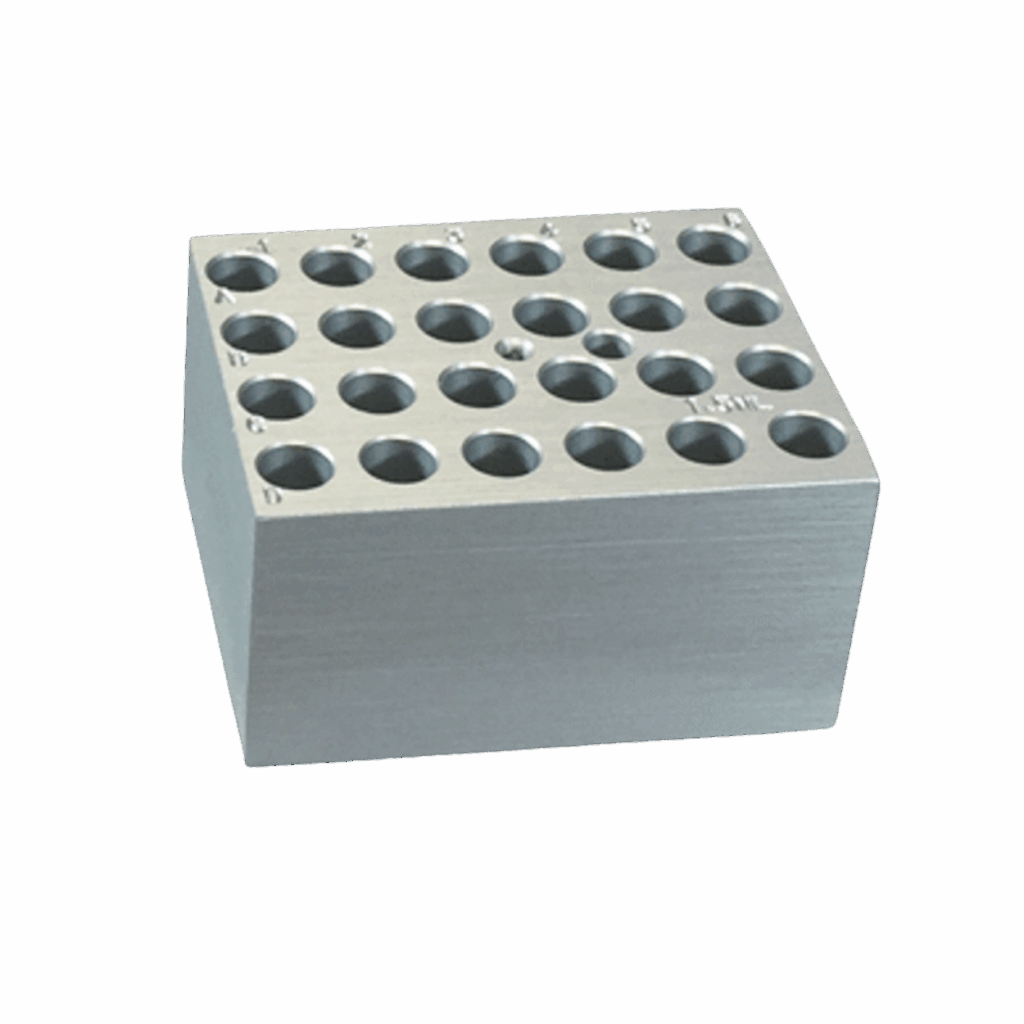
Load dPCR plate

Digital reaction and data collection
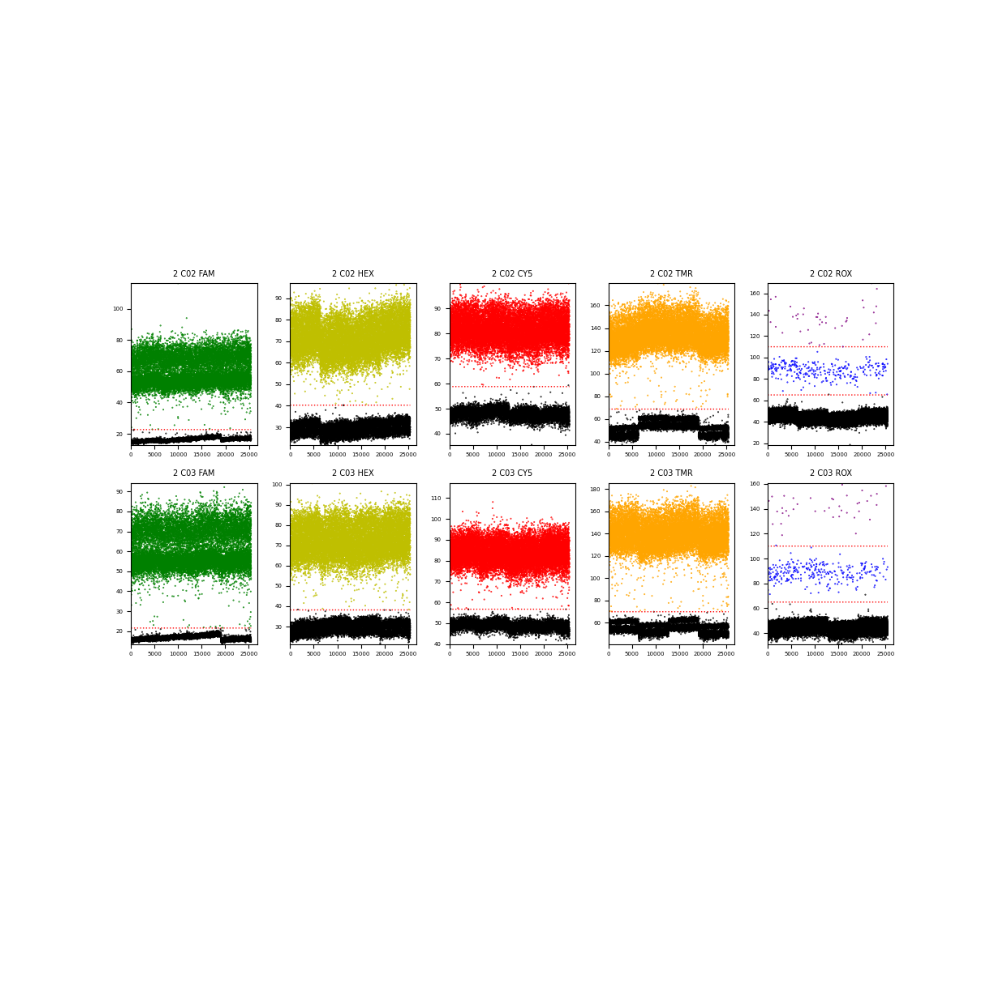
Data Analysis
Performance of Atila Multiplex Digital PCR Quantification Assay
Data Shows:
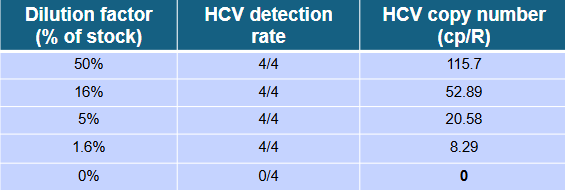
1) Testing of a patient’s HCV positive plasma sample diluted in negative matrix on Qiagen QiAcuity digital PCR platform.
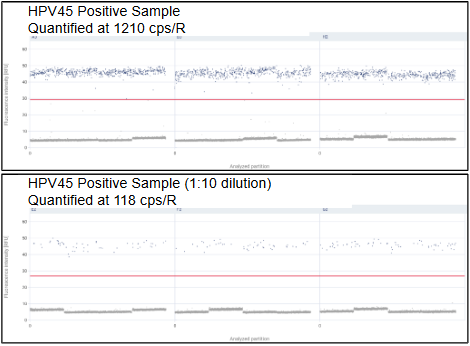
(2) 1D Scatter Plot For HPV45 Positive Samples Demonstrating Ideal Linearity
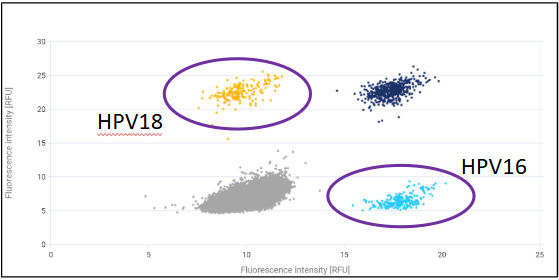
(3) 2D Scatter Plot of A Multi Positive Sample Analyzed on dPCR(this sample is co-infected with HPV16 and HPV18)
Available Infectious Disease Testing Products (click to see targets)
Respiratory syncytial virus, Influenza A, Influenza B, SARS-CoV-2, Rhinovirus, Enterovirus, Coronavirus (229E, HKU1, OC43, NL63), Parainfluenza virus (1-4), Human adenovirus, Human bocavirus, Human metapneumovirus, Mycoplasma pneumonia, Klebsiella pneumonia, Bordetella parapertussis, Bordetella pertussis, Streptococcus pneumonia, Haemophilus influenzae, Legionella pneumophila, Chlamydia pneumonia
SARS-CoV-2 (N and ORF gene without differentiation).
SARS-CoV-2 (N and ORF gene without differentiation)
SARS-CoV-2, Influenza, A Influenza B
Influenza A, Influenza B, Respiratory syncytial virus (RSV)
Acinetobacter baumannii, Citrobacter spp., Enterobacter cloacae, Enterococcus spp.,, Escherichia coli, Klebsiella aerogenes, Klebsiella oxytoca, Klebsiella pneumoniae, Morganella morganii, Proteus spp., Providencia stuartii, Pseudomonas aeruginosa, Staphylococcus saprophyticus, Streptococcus agalactiae, Candida albicans
Bacteria- Acinetobacter baumannii, Anaerococcus spp,, Bacteroides fragilis, Citrobacter spp., Enterococcus spp., Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Proteus spp., Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus agalactiae, Streptococcus pyogenes
Virus- Herpes simplex virus
Fungi- Candida spp.. Candida glabrata, Candida krusei
Trichophyton spp., Aspergillus, Curvularia spp., Fusarium solani, Epidermophyton floccosum, Cryptococcus, Malassezia spp., Sarocladium strictum, Scopulariopsis brevicaulis. Microsporum audouinii/canis Alternaria, NeoScytalidim d/h, Geotrichum candidum, Candida spp., Trichosporon Neofusicoccum mangiferae
Bacteria- Bacterial Vaginosis Associated Bacteria-2, Megasphaera-1 Megasphaera-2, Trichomonas vaginalis, Atopobium vaginae
Fungi- Candida albicans, Candida dubliniensis, Candida tropicalis, Candida parapsilosis, Candida krusei, Candida glabrata
KPC, NDM, OXA-48, IMP, VIM, CTX, VanA, VanB, MecA, SUL1, SUL2, SUL3, Qnrs, dfrA1, dfrA5, dfrA12, dfrA17 MefA, MrsA, ermA, ermB, ermC, mphA, and ereA
Candida auris
HPV16, HPV18/45, HPV31/33/35/52/58 and HPV39/51/56/59/68
HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 and simultaneously identify HPV16 and HPV18.
HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68
Detects Monkeypox virus (MPXV) in subtypes from the West African clade and the Congo Basin (Central African) clade
Plasmodium falciparum, Plasmodium knowlesi ,Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax
Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium africanum, Mycobacterium canettii and Mycobacterium microti
H5N1
Treponema pallidum subspecies pallidum
Panel Pathogens- Escherichia coli,,Enterococcus faecalis,, Candida spp.2, Serratia marcescens, Enterococcus faecium, Candida krusei, Staphylococcus epidermidis, Enterococcus gallinarum, Candida glabrata, Staphylococcus aureus, Stenotrophomonas maltophilia, Streptococcus pneumoniae, Klebsiella pneumoniae, Streptococcus agalactea, Enterobacter cloacae, Klebsiella oxytoca, Acinetobacter baumannii, Haemophilus influenzae, Klebsiella aerogenes, Pseudomonas aeruginosa, Neisseria meningitidis, Bacteroides fragilis, Streptococcus pyogenes, Proteus mirabilis, Candida auris, Staphylococcus lugdunensis, Cryptococcus neoformans gattii, Listeria monocytogenes, Salmonella spp.1 Streptococcus mitis
Drug Resistance Markers- CTX, OXA-48, IMP, NDM, VIM, MecA, KPC, VanA, VanB, TEM, MCR-1
1 Primer design is based on the entire genus
2 Candida albicans, Candida tropicalis, Candida parapsilosis, and Candida dubliniensis.
Streptococcus pyogenes
Pathogens: H. influenzae, L. monocytogenes, N. meningitidis, S. agalactiae, S. pneumoniae, E. coli, C. neoformans
Drug resistance gene markers: carbapenemase genes (NDM, KPC, OXA-48, VIM, IMP), extended spectrum beta-lactamase (ESBL) gene (CTX-M1), vancomycin resistance genes (VanA, VanB), oxacillin/methicillin resistance gene (MecA), sulfanamide resistant genes (SUL1, SUL2, SUL3), trimethoprim resistant genes (dfrA1, dfrA5, dfrA12, dfrA17), plasmid-mediated quinolone resistance marker (QnrS), and Marcolide resistant genes (MefA, MrsA, ermA, ermB, ermC, ereA, mphA).
Streptococcus pneumoniae (S. pneumoniae)
Streptococcus agalactiae (S. agalactiae)
Herpes simplex virus type 1 and 2
Candida albicans, Candida dubliniensis, Candida tropicalis, Candida parapsilosis, Candida glabrata, and Candida krusei
Gardnerella vaginalis (GV), Atopobium vaginae (AV), Bacterial Vaginosis Associated Bacteria-2 (BVAB-2), Megasphaera-1 and Megasphaera-2 (Mega1 and Mega2)
Ureaplasma urealyticum (UU), Ureaplasma parvum (UP)
Mycoplasma hominis
Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, Mycoplasma genitalium
ATWH CT/NG/TV/MG Detection Kit: Chlamydia trachomatis, Trichomonas vaginalis, Mycoplasma genitalium, Neisseria gonorrhoeae
ATWH HSV 1/2 Detection Kit: Herpes Simplex Virus type 1, Herpes Simplex Virus type 2
ATWH Candidiasis Detection Kit: Candida albicans, Candida dubliniensis, Candida tropicalis, Candida parapsilosis, Candida glabrata, Candida krusei
ATWH MH Detection Kit: Mycoplasma Hominis
ATWH Ureaplasma Detection Kit: Ureaplasma urealyticum, Ureaplasma parvum
ATWH Bacterial Vaginosis Detection Kit: Gardnerella vaginalis, Atopobium vaginae, Bacterial Vaginosis Associated Bacteria-2, Megasphaera-1, Megasphaera-2
ATWH Internal Control Detection Kit: Homo Sapiens
gyrA gene with S91F mutation from Neisseria gonorrhea
mutations at positions 2058 and 2059 in the 23S rRNA gene (A2058G, A2059G, A2058T, A2058C, and A2059C, Escherichia coli numbering) from Mycoplasma genitalium (MG)
Chlamydia trachomatis
Human Immunodeficiency Virus
Hepatitis B Virus
Hepatitis C Virus
Available Oncology Testing Products (click to see targets)
Available Reproductive Health Testing (click to see targets)
Exon 4, exon 4 pseudogene form, exon 5 and exon 7 on chromosome 1.
Trisomy 21, Trisomy 18, Trisomy 13, Fetal Gender, Fetal Fraction.
Trisomy 21, 18, 13, sex chromosome aneuploidy, fetal fraction, and gender determination.
Fetal Fraction
Genomic DNA contamination