ScreenFire HPV RS Assay
The ScreenFire HPV RS Kit is an extraction-free, in vitro nucleic acid isothermal amplification assay with real-time fluorescence detection, designed for the qualitative detection of DNA from 13 high-risk Human Papillomavirus (HPV) genotypes. The assay identifies HPV16, HPV18/45, HPV31/33/35/52/58, and HPV39/51/56/59/68 in groups of risk stratification for cancers associated with these high-risk HPV types.
Each kit includes 100 tests.
This product is CE-IVD certified. It is for research use only in US.
ScreenFire HPV RS Assay
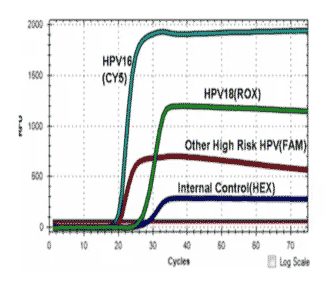
The ScreenFire HPV RS Assay is an isothermal nucleic acid amplification assay for the qualitative detection of high-risk types of human papillomavirus (HPV). High-risk HPV specific primers and fluorescent probes are used to amplify regions of viral genomic DNA including E6/E7 regions under isothermal conditions.
The assay detects HPV types 16 alone, 18/45 as a group, 31/33/35/52/58 as a group (alpha-9 HPV group) and 39/51/56/59/68 (other high-risk HPV group) as a group.
Human papillomavirus (HPV) is the most common sexually transmitted virus in the world. In most cases, HPV infections are transient, and the body will clear the virus on its own. In some instances, however, the virus is persistent and will cause normal cells to become abnormal, leading to cancer.
These oncogenic types of HPV are classified as high risk and include HPV 16 and 18. Other types are considered low risk, as they do not appear to cause cancer. According to the World Health Organization (WHO), HPV is the second biggest cause of female cancer mortality worldwide, claiming about 250000 lives annually.
In Europe alone, the disease claims about 15000 lives each year. It is estimated that nearly 70% of cervical cancers are caused by HPV types 16 and 18.
Features Include
Fast Speed
Easy To Use
Flexibility
Low Cost
Technical Specs
Targets
Reaction Time
Sample Types
Sensitivity
Instruments
Workflow Overview for the ScreenFire HPV RS Assay
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.