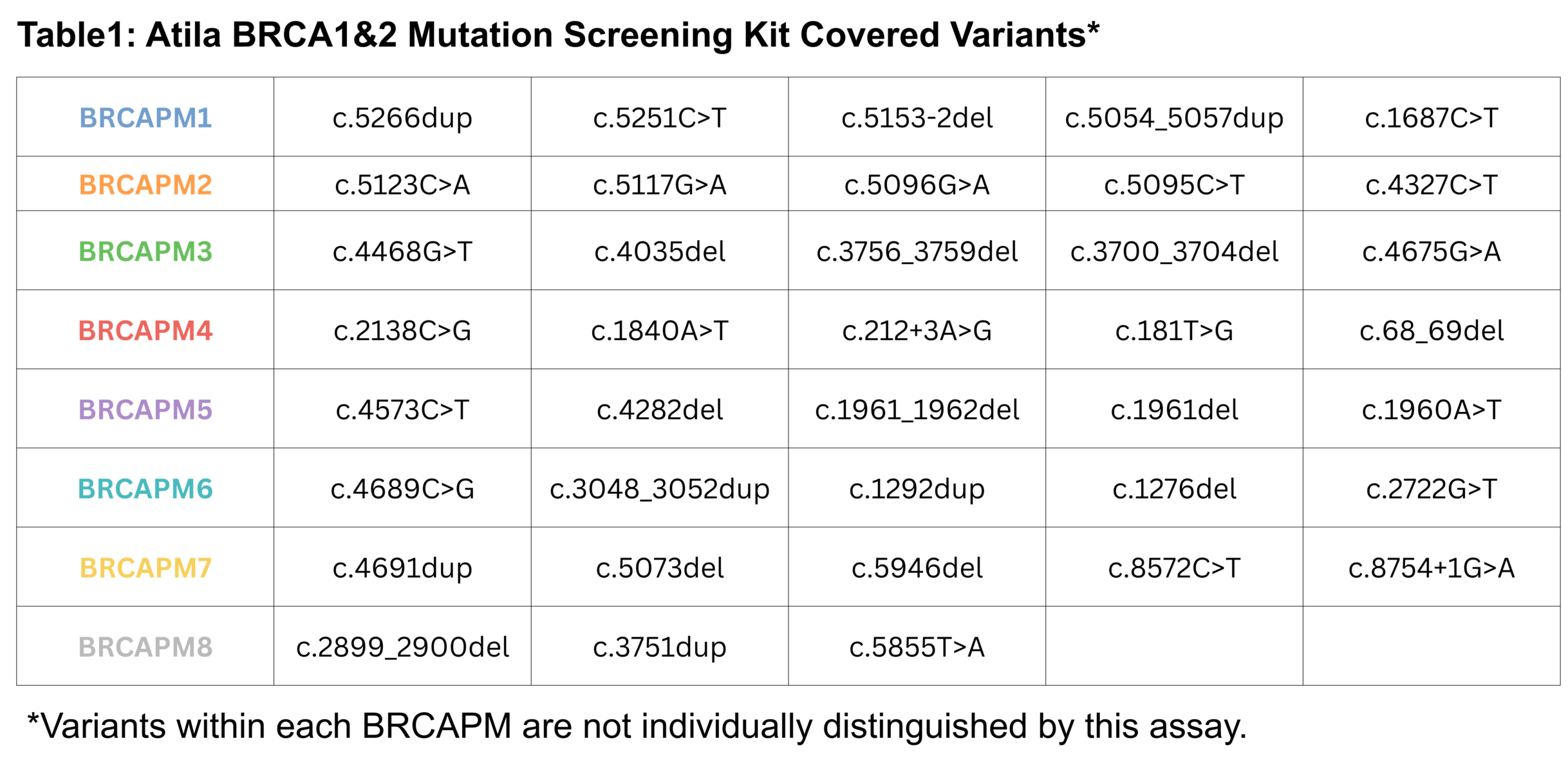
NRAS Multiplex Mutation Screening Kit
The NRAS gene belongs to a class of genes known as oncogenes. When mutated, oncogenes have the potential to cause normal cells to become cancerous. Somatic mutations in the NRAS gene are involved in the development of several types of cancer.
These mutations lead to an NRAS protein that is constitutively active and can direct cells to grow and divide without control. Studies suggest that NRAS gene mutations are common in the aggressive skin cancer melanoma, including individuals without giant congenital melanocytic nevus.
Cancer is a genetic disease with uncontrolled growth of abnormal cells. Some types of cancer are frequently associated with specific genetic mutations. Those mutations provide the biomarkers that allow more specific cancer treatments, known as targeted therapy.
Targeted therapy is a newer type of cancer treatment that offers patients the opportunity to use a drug that has a greater effect on cancerous tissue, reducing many of the side effects associated with standard therapy. Therefore, it is a critical step towards targeted therapy by identifying the cancer mutations with a precise and sensitive method.
Features Include
Technical Specs
Workflow Overview for the NRAS Multiplex Mutation Screening Kit
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.

DNA Extraction

PCR Reaction Assembly
Digital PCR