iSAFE™ NIPT Genomic DNA Contamination Determination Assay Kit
The iSAFETM NIPT Genomic DNA Contamination Determination Assay Kit is intended to test the possible blood cell genomic DNA contamination in the purified cfDNA from the maternal blood.
Each kit includes 100 tests.
This product is for research use only.
iSAFE™ NIPT Genomic DNA Contamination Determination Assay Kit
The iSAFE™ NIPT Genomic DNA Contamination Determination Assay Kit measures the amount of genomic DNA in the extraction cfDNA and provides an important quality control factor for the Non-invasive prenatal testing (NIPT) results.
Whole blood transportation and storage are important factors during the sample processing for cfDNA extraction. Improper handling of the blood specimen can result in lysis of nucleated blood cells and contamination of cfDNA by the blood cell genomic DNA.
Features Include
Technical Specs
Workflow Overview for the iSAFE™ NIPT Genomic DNA Contamination Determination Assay Kit
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.

Collect maternal blood sample.

dPCR reaction assembly.
Digital PCR.
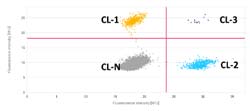
Data analysis.
NIPT Safety Data Sheet
Discover our comprehensive Safety Data Sheet (SDS) for Noninvasive Prenatal Testing (NIPT), providing essential information on hazards, handling, emergency procedures, and regulatory compliance to ensure safety and peace of mind.
Atila BioSystems prioritizes the safety and well-being of our clients and partners.

