iSAFE™ NIPT Gender and Fetal Fraction Determination Assay Kit
The iSAFE NIPT Gender and Fetal Fraction Determination Assay Kit can simultaneously quantify cfDNA, determine fetal gender, and measure fetal fraction for male fetus in the maternal blood cfDNA. When performed separately, the quantification derived from this assay can be used for NIPT assay.
Each kit includes 100 tests.
This product is for research use only.
iSAFE™ NIPT Gender and Fetal Fraction Determination Assay Kit
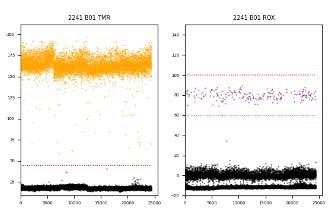
The iSAFE™ NIPT Gender and Fetal Fraction Determination Assay Kit can detect fetal gender as early as 6 weeks. It is the first digital PCR-based gender determination assay with improved accuracy than the standard qPCR technology.
In addition, the kit also measures the fetal fraction (FF) in the maternal blood cfDNA of male fetus and provides DNA quantification information. The FF can be used as an important quality control factor of the Non-invasive prenatal testing (NIPT) results.
Features Include
Technical Specs
Workflow Overview for the iSAFE™ NIPT Gender and Fetal Fraction Determination Assay Kit
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.

Collect maternal blood sample.

dPCR reaction assembly.
Digital PCR.