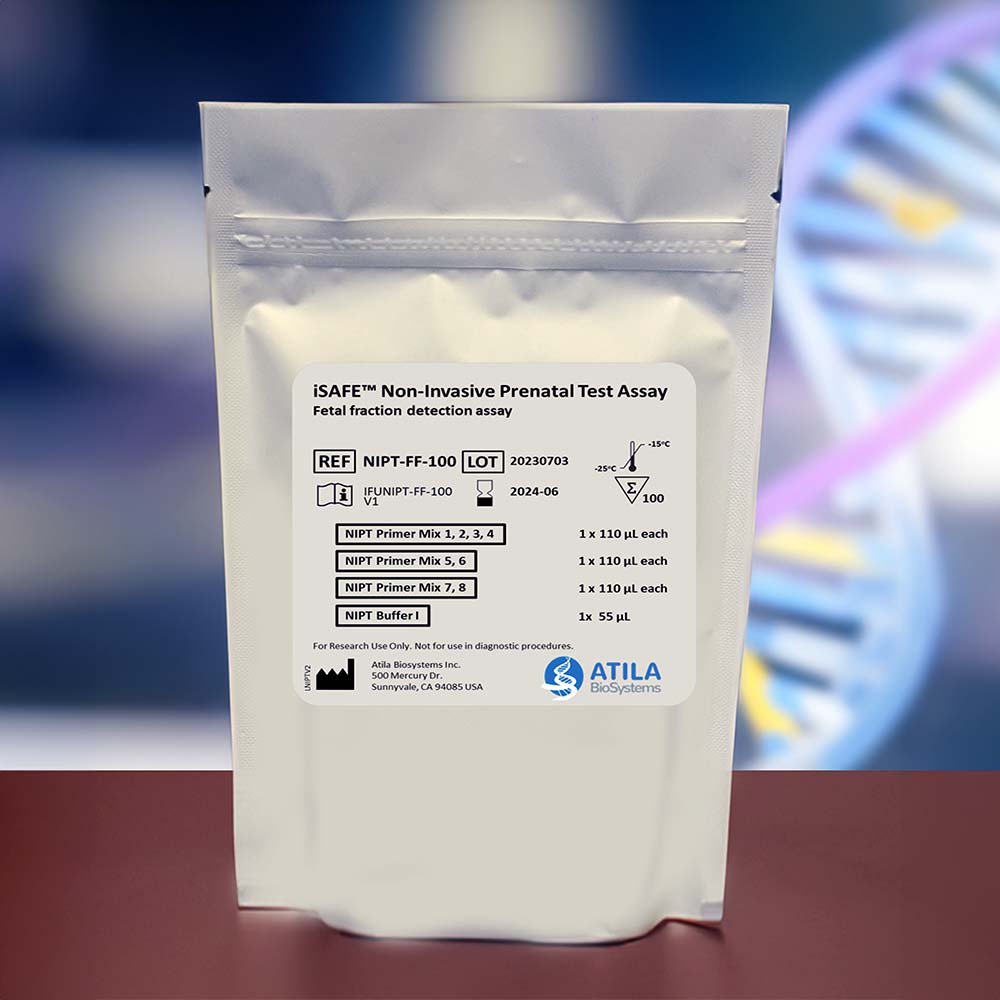
iSAFE™ NIPT Fetal Fraction Determination Assay Kit
The iSAFE NIPT Fetal Fraction Determination Assay Kit can simultaneously quantify cfDNA and measure fetal fraction in the maternal blood cfDNA. When performed separately, the quantification derived from this assay can be used for NIPT assay.
Each kit includes 100 tests.
This product is for research use only.
iSAFE™ NIPT Fetal Fraction Determination Assay Kit
The iSAFE™ NIPT Fetal Fraction Determination Assay Kit is a standalone kit that measures the FF in the maternal blood cfDNA and provides DNA quantification information.
The fetal fraction (FF) is the percentage of total maternal blood cell-free DNA (cfDNA) that is of fetoplacental origin. It is often considered as an important quality control factor of the Non-invasive prenatal testing (NIPT) results.
Features Include
Technical Specs
Workflow Overview for the iSAFE™ NIPT Fetal Fraction Determination Assay Kit
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.

Collect maternal blood sample.

dPCR reaction assembly.
Digital PCR.