iSAFE™ NIPT Assay on naica® System
Atila BioSystem and Stilla Technology co-branded non-invasive prenatal test assay, including Trisomy 21, Trisomy 18, Trisomy 13, Fetal Fraction and Gender Determination. Compatible with Stilla Technologies’ naica system.
Each kit includes 100 tests.
This product is for research use only.
iSAFE™ NIPT Assay on naica® System
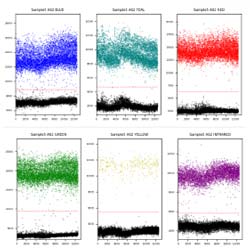
The iSAFE™ NIPT Assay on naica® System (NIPT) screens for aneuploidy of chromosomes 21 (Down Syndrome), 18 (Edwards Syndrome), and 13 (Patau Syndrome) in singleton pregnancies. The assay applies Crystal Digital PCR™ technology on the 6-color naica® system to accurately quantify the copy numbers of chromosome 21, 18, and 13 in maternal blood cell-free DNA (cfDNA).
The assay also quantifies the copy number of a fetal specific target for fetal fraction calculation and quantifies the copy number of chromosome Y for gender determination.
Features Include
Technical Specs
Workflow Overview for the iSAFE™ NIPT Assay on naica® System
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.

Collect maternal blood sample.

dPCR reaction assembly.

Load dPCR chips.

dPCR and data collection.