iAMP MH Detection Kit
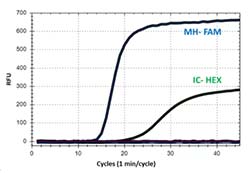
The iAMP MH Detection Kit is intended for use to qualitatively detect Mycoplasma hominis DNA present in vaginal or cervical swabs from symptomatic or asymptomatic females.
Mycoplasma hominis plays essential roles in pathogenicity and directly correlated with the health of host immune system. Mycoplasma hominis has been associated with a number of clinically significant infections, ranging from pelvic inflammatory diseases, chorioamnionitis, postpartum endometritis bacterial vaginosis, arthritis, osteoarthritis, wound infections, and several conditions in neonates.
Mycoplasma hominis can transfer from one person to another by sexual contact with an infected person and can also be passed from mother to baby during delivery. Pregnant women with this infection may have high risk of ectopic pregnancy, miscarriage, and early birth.
Features Include
Technical Specs
Workflow Overview for the iAMP MH Detection Kit
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.
Dry swab in sample tube.

Add up to 750µL 1X Atila sample buffer.

Vortex and incubate at 95C for 10 minutes.
Add 20 µL reaction mix and 5 µL processed sample.

Run on standard PCR instrument.