iAMP MG Resistance Assay
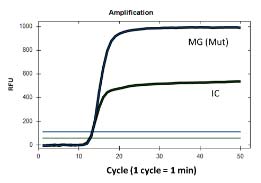
The iAMP MG Resistance Assay is an extraction-free real-time fluorescent isothermal PCR assay based on Atila’s proprietary isothermal amplification technology intended for the qualitative detection of mutations at positions 2058 and 2059 in the 23S rRNA gene (A2058G, A2059G, A2058T, A2058C, and A2059C, Escherichia coli numbering) that are associated with susceptibility or resistance to azithromycin (macrolide-based antibiotic) in Mycoplasma genitalium (MG).
Each kit includes 100 tests.
This product is CE-IVD certified. It is for research use only in US.
iAMP MG Resistance Assay
The iAMP MG Resistance Assay is a real-time fluorescent isothermal PCR assay based on Atila’s proprietary isothermal amplification technology intended for the qualitative detection of mutations at positions 2058 and 2059 in the 23S rRNA gene (A2058G, A2059G, A2058T, A2058C, and A2059C, Escherichia coli numbering) that are associated with susceptibility or resistance to azithromycin (macrolide-based antibiotic) in Mycoplasma genitalium (MG).
Mycoplasma genitalium (or Mgen) is a sexually transmitted bacterium that can cause reproductive tract infections of the penile urethra or cervix. Mgen causes symptomatic and asymptomatic urethritis. It may also play a role in cervicitis, pelvic inflammatory disease (PID), preterm delivery, spontaneous abortion, and infertility.
Azithromycin regimens have been commonly used for treatment of M. genitalium infections. However, failure of azithromycin treatment has been reported in cases of M. genitalium–positive nongonococcal urethritis (NGU), and macrolide resistant strains of M. genitalium have been isolated from case-patients worldwide.
Features Include
Technical Specs
Workflow Overview A
Workflow Overview for the iAMP MG Resistance Assay
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.

Left over sample of MG positive sample.

Thaw at room temperature for 10 minutes.

Vortex.
Add 20 µL reaction mix and 5 µL processed sample.

Run on standard PCR instrument.
From sample to answer in less than 90 minutes.
Workflow Overview B
Workflow Overview for the iAMP MG Resistance Assay
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.

Centrifuge at 10,000 RPM for 15 min, remove the supernatant completely.

Add 120 µL Atila 1x sample buffer and vortex thoroughly.

Incubate at 95C for 10 minutes.

Add 20 µL reaction mix and 5 µL processed sample.

Run on standard PCR instrument.

From sample to answer in less than 90 minutes.
Workflow Overview C
Workflow Overview for the iAMP MG Resistance Assay
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.
Dry swab in sample tube.

Add up to 750µL Atila 1x sample buffer.

Vortex & incubate at 95C for 10 minutes.

Add 20 µL Reaction Mix and 5 µL processed sample.

Run on standard PCR instrument.


