iAMP Drug Resistance Panel
The iAMP Drug Resistance Panel is an extraction-free real-time fluorescent isothermal PCR assay based on Atila’s proprietary isothermal amplification technology intended for the qualitative detection of drug resistance (DR) gene markers.
iAMP-DRFP-100 (Liquid Format), iAMP-DRFP-96 (Zebra BioDome Format)
This product is research use only in the US.
iAMP Drug Resistance Panel
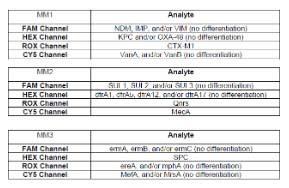
The iAMP Drug Resistance Panel is a real-time fluorescent isothermal PCR assay based on Atila’s proprietary isothermal amplification technology intended for the qualitative detection of drug resistance (DR) gene markers including carbapenemase genes (NDM, KPC, OXA-48, VIM, IMP), extended spectrum beta-lactamase (ESBL) gene (CTX-M), vancomycin resistance genes (VanA, VanB), oxacillin/methicillin resistance gene (MecA), sulfanamide resistant genes (SUL1, SUL2, SUL3), trimethoprim resistant genes (dfrA1, dfrA5, dfrA12, dfrA17), plasmid-mediated quinolone resistance marker (QnrS), and Marcolide resistant genes (MefA, MrsA, ermA, ermB, ermC, ereA, mphA) in clinician-collected or self-collected clean-catch urine specimens obtained from individuals with or suspected of urinary tract infections (UTI), or clinician-collected wound swab specimens obtained from individuals with signs and symptoms of wound infections.
To combat and control the spread of resistant pathogens, fast, robust and affordable antimicrobial resistance assays are highly desired. To fulfill this unmet need, Atila Biosystems applies innovative technologies to the development of iAMP Drug Resistance Panel, allowing laboratories to detect drug-resistant bacteria with the speed, simplicity, and reasonable cost.
Antimicrobial resistance is an urgent global public health threat. Infections caused by antibiotic-resistant bacteria are difficult to treat, leading to longer hospital stays, higher medical costs and increased mortality. In 2019, antimicrobial resistance killed at least 1.27 million people worldwide and associated with nearly 5 million deaths. In the U.S., more than 2.8 million antimicrobial-resistant infections occur each year. More than 35,000 people die as a result, according to CDC’s 2019 Antibiotic Resistance (AR) Threats Report.
Antibiotic resistance occurs naturally, but misuse and overuse of antibiotics in humans and animals is accelerating the process. According to the WHO, antibiotic resistance is increasing to dangerously high levels worldwide. In the absence of a comprehensive effort to combat emerging drug resistance, infections due to multi-drug resistant organisms are predicted to be the leading cause of death worldwide by 2050.
Features Include
Technical Specs
Workflow Overview A
Workflow Overview for the iAMP Drug Resistance Panel
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.

Left over sample of UTI or wound sample.

Thaw at room temperature for 10 minutes.

Vortex & incubate at 95C for 10 minutes.
Add 20 µL Reaction Mix and 5 µL processed sample.

Run on standard PCR instrument.
From sample to answer in less than 90 minutes.
Workflow Overview B
Workflow Overview for the iAMP Drug Resistance Panel
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.

20uL 1xAtila sample buffer mixed with 20uL urine specimen.

Vortex and centrifuge.

Incubate at 95C for 10 minutes.

Add 20 µL Reaction Mix and 5 µL processed sample.

Run on standard PCR instrument.

From sample to answer in less than 90 minutes.
Workflow Overview C
Workflow Overview for the iAMP Drug Resistance Panel
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.
Dry swab in sample tube.

Add up to 750µL 1X Atila sample buffer.

Vortex & incubate at 95C for 10 minutes.

Add 20 µL Reaction Mix and 5 µL processed sample.

Run on standard PCR instrument.


