iAMP COVID-19 SANO Assay
This extraction-free kit specifically detects RNA and later cDNA from the N and ORF-1ab genes of the SARS-CoV-2 virus in saliva, nasal, nasopharyngeal and/or oropharyngeal swabs from patients with signs and symptoms of infection who are suspected of COVID-19.
Each kit includes 100 tests.
This product is CE-IVD certified. It is for research use only in US.
iAMP COVID-19 SANO Assay
The COVID-19 Pandemic has highlighted the importance of rapid and accurate diagnostic assays. The iAMP COVID-19 SANO Assay is a real-time reverse transcription isothermal amplification test based on a proprietary isothermal amplification technology termed OMEGA amplification (Patent: WO 2017/205510 A1; publication: The Journal of Molecular Diagnostics, Vol.22, No 3, 419-428, 2020).
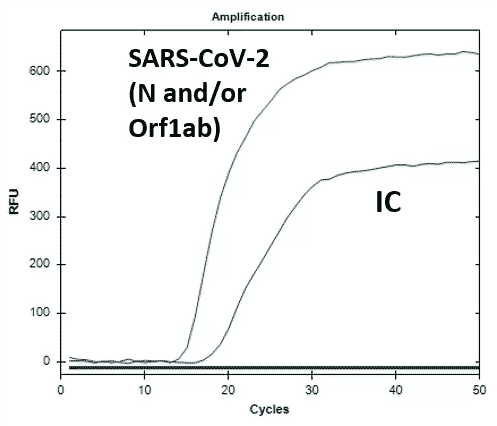
OMEGA primer sets are designed to specifically detect RNA and later cDNA from the N and ORF-1ab genes of the SARS-CoV-2 virus in saliva, nasal, nasopharyngeal and/or oropharyngeal swabs from patients with signs and symptoms of infection who are suspected of COVID-19. The iAMP COVID-19 SANO Assay’s key differentiator from current rRT-PCR COVID-19 assays is its ability to detect SARS-CoV-2 RNA directly from samples without prior RNA extraction process.
Swab specimens stored in Atila Elution Solution buffer or commonly used transport medium (saline, PBS, or viral transport medium) can be directly used for OMEGA isothermal amplification and signal detection. Sample to result takes about 1 hour. Hands-on time is less than 5 minutes per sample.
Coronavirus disease 2019 (COVID-19) is a disease caused by a virus named severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). It is highly contagious and spreads quickly, Although many people with COVID-19 have mild to moderate symptoms and can recover on their own, COVID-19 can be serious illness and lead to death in some people such as older adults and people with pre-existing medical conditions. As of November 2023, there have been 772 million confirmed cases of COVID-19, including 7 million deaths, reported to WHO.
Features Include
Fast Speed
Easy To Use
Flexibility
Low Cost
Technical Specs
Assay Targets
Reaction Time
Sample Types
Sensitivity
Instruments
Workflow Overview for the iAMP COVID-19 SANO Assay
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.