iAMP COV2/INF Detection Kit
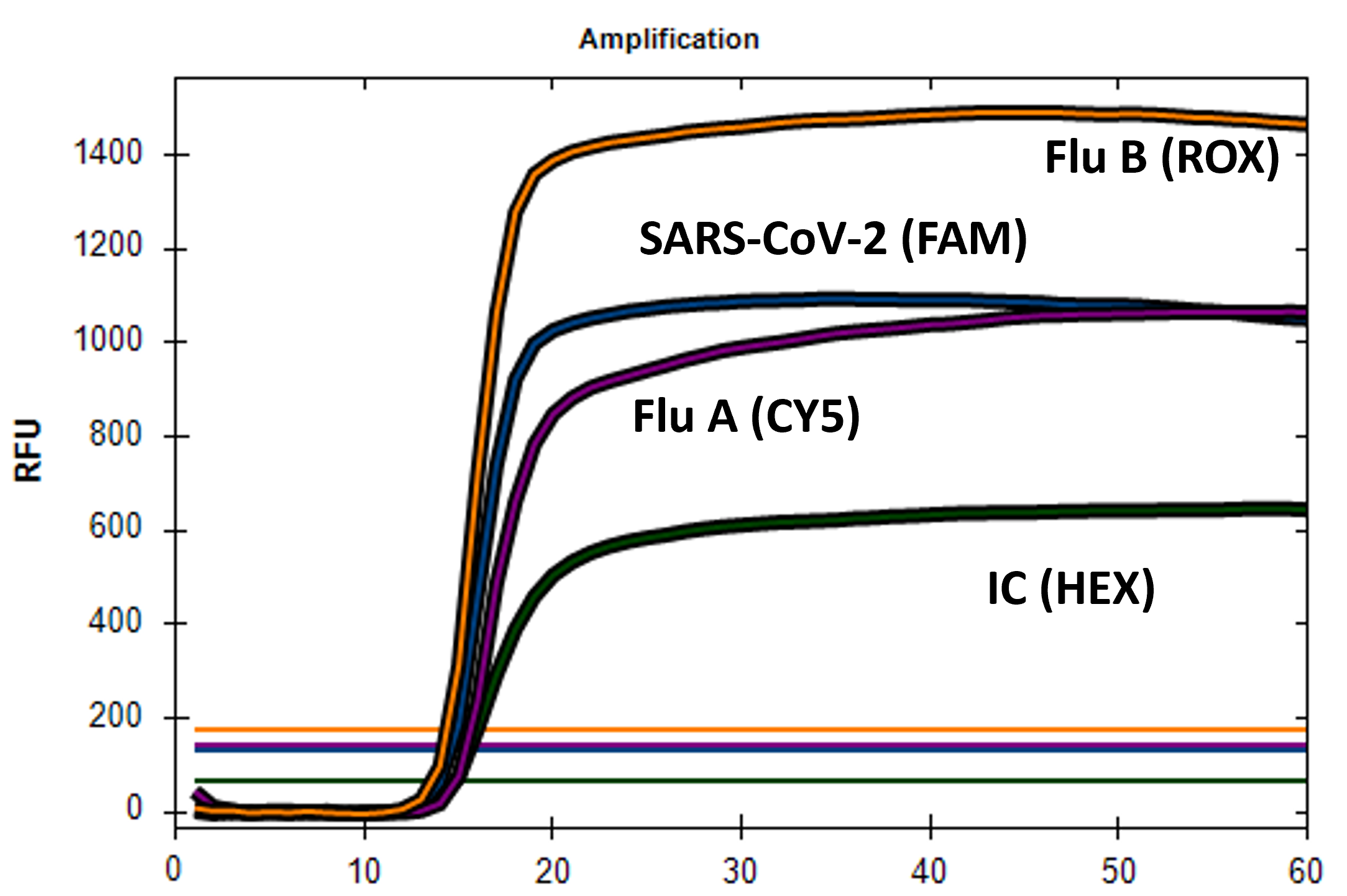
Our iAMP COV2/INF Detection Kit uses a simple, extraction-free workflow to deliver results in under 90 minutes. This kit is intended to detect and differentiate influenza A, B, and SARS-CoV-2 in a single assay from a single swab. As low as 12.5 copies of SARS-CoV-2, and 50 copies of genomic RNA can be detected respectively in a reaction.
The symptoms alone cannot tell the difference between the flu and COVID-19. Highly specific testing is therefore important to diagnose the illness. Current approach requires nucleic acid extraction from the viruses.
Influenza (flu) and COVID-19 are contagious respiratory diseases which posse similar symptoms. Both diseases can result in severe complications or even fatal. Infection of SARS-CoV-2 virus causing COVID-19 is more concerning due to its longer incubation period in host, highly contagious, and cause more severe symptoms.
The virus is also constantly changing its genomic code as part of its evolution causing reinfection. The COVID-19 outbreak has so far resulted in at least 1M deaths in the United States alone.
Features Include
Technical Specs
Workflow Overview for the iAMP COV2/INF Detection Kit
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.
Dry swab in sample tube.

Add up to 1000µL 1X Elution solution (1XES).

Vortex briefly (2-3 sec) and incubate for 5 min to hydrate the swabs.
Add 20 µL Reaction Mix and 5 µL processed sample.

Run on standard PCR instrument.