iAMP® Candidiasis Detection Kit
This extraction-free kit qualitatively detect 6 Candida spp. causing Candidiasis including Candida albicans, Candida dubliniensis, Candida tropicalis, Candida parapsilosis, Candida glabrata, and Candida krusei in vaginal/cervical swab samples.
iAMP-CAN-100 (Liquid Format). This product is CE-IVD certified but RUO in the US. Each kit includes 100 tests.
iAMP-CAN-96 (Zebra BioDome Format). This product is RUO. Each kit includes 96 tests.
iAMP® Candidiasis Detection Kit
The iAMP® Candidiasis Detection Kit is intended for use to qualitatively detect 6 Candida spp. causing VVC: Candida albicans, Candida dubliniensis, Candida tropicalis, Candida parapsilosis, Candida glabrata, and Candida krusei in vaginal/cervical swab samples.
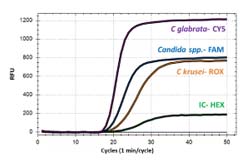
The kit simultaneously identifies Candida glabrata and Candida krusei from the rest of 4 Candida spp. to provide additional information for the treatment. Based on our isothermal technology, the Candidiasis detection kit features a very simple procedure without the need of DNA extraction or purification, a rapid detection of less than 90 minutes and superior sensitivity and specificity.
Vulvovaginal candidiasis (VVC) occurs as a result of displacement of the normal vaginal flora by species of the fungal genus Candida. The usual symptoms include irritation, itching, burning with urination and thick whitish discharge which are commonly found in other vaginal infections as well. Therefore, a laboratory test is needed to distinguish vaginal candidiasis for appropriate treatment.
VVC can be classified based on severeness of the symptoms, recurrence frequency and level of resistance to antifungal agents. Different species have varying level of susceptibility to –azole antifungal agents making it crucial to differentiate between them. For example, several research has shown that C glabrata isolates demonstrated decreased in vitro susceptibility to fluconazole whereas C krusei is considered intrinsically resistant to it.
Features Include
Technical Specs
Workflow Overview for the iAMP® Candidiasis Detection Kit
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.
Dry swab in sample tube.

Add up to 750µL 1X Atila sample buffer.

Vortex & incubate at 95C for 10 minutes.
Add 20 µL Reaction Mix, and 5 µL processed sample.

Run on standard PCR instrument.