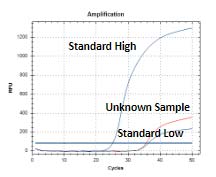
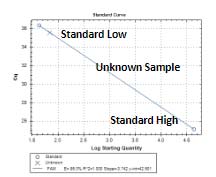
Atila HCV Assay Kit
The Atila HCV Assay is a real-time reverse transcription assay test for the in vitro quantitative detection of human hepatitis C virus (HCV) RNA in human plasma or serum. This test is not intended for use as a screening test for HCV or an aid in diagnosis of infection with HCV.
The Atila HCV Assay Kit is intended for use by qualified and trained laboratory personnel specifically instructed and trained in the techniques of real-time nucleic acid amplification and in vitro diagnostic procedures.
Each kit includes 100 tests.
This product is for research use only.
Atila HCV Assay Kit
The Atila HCV Assay Kit is a reverse transcription polymerase chain reaction (RT-PCR) assay based on a proprietary technology developed to improve the efficiency of detecting multiple viral nucleic acid targets simultaneously by reducing non-specific interactions and amplifications.
DNA oligos are designed to specifically detect and discriminate HCV RNA. The assay does not discriminate the HCV genotypes. The test also includes an Internal Control (IC) for monitoring test performance in each individual test.
The Atila HCV Assay Kit is intended for use by qualified and trained laboratory personnel specifically instructed and trained in the techniques of real-time nucleic acid amplification and in vitro diagnostic procedures.
Hepatitis C virus (HCV) is a single-stranded RNA virus, with a genome size of approximately 9500 nucleotides. HCV can cause Hepatitis C, a liver disease, and is a major cause of liver cancer. The virus causes both acute and chronic hepatitis. Around 30% (15–45%) of infected persons spontaneously clear the virus within 6 months of infection without any treatment.
However, the remaining 70% (55–85%) of persons will develop chronic HCV infection. Of those with chronic HCV infection, the risk of cirrhosis ranges between 15% and 30% within 20 years. The HCV is a bloodborne virus, is transmitted by sharing injection equipment, transfusion or exposure to the infected blood, and can also be transmitted sextually or from infected mother to baby.
Features Include
Technical Specs
Workflow Overview for the Atila HCV Assay Kit
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.
Collect plasma sample.

Add 1/10 elution volume of processing control (5uL) to plasma sample.

Mix 12.5 µL extracted sample with 12.5 µL reaction mix in a new tube.
Load mix into PCR plate wells.

Transfer the reaction mix to a PCR machine.