Atila Blood Screening Assay Kit
The Atila Blood Screening Assay is a real-time reverse transcription assay test for the in vitro qualitative detection of Human Immunodeficiency Virus Type 1 (HIV-1) Group M RNA, HIV-1 Group O RNA, Hepatitis C Virus (HCV) RNA, and Hepatitis B Virus (HBV) DNA in human plasma. The test can be used to screen for HIV-1 Group M RNA, Group O RNA, HCV RNA, and HBV DNA in plasma specimens from individual human donors of whole blood, blood components, and source plasma. This test is not intended for use as an aid in diagnosis of infection with HIV, HCV, or HBV.
Each kit includes 100 tests.
This product is for research use only.
Atila Blood Screening Assay Kit
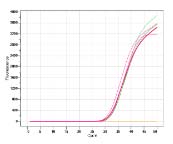
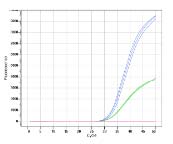
The Atila Blood Screening Assay Kit is a multiplex reverse transcription polymerase chain reaction (RT-PCR) assay based on a proprietary technology developed to improve the efficiency of detecting multiple viral nucleic acid targets simultaneously by reducing non-specific interactions and amplifications.
Multiple sets of DNA oligoes are designed to specifically detect and discriminate the HIV-1 RNA, HBV DNA and HCV RNA. The assay doesn’t discriminate the HIV-1 groups, HBV genotypes, or the HCV genotypes. The test also includes an Internal Control (IC) for monitoring test performance in each individual test.
Human Immunodeficiency Virus Type 1 (HIV-1), Hepatitis C Virus (HCV) and Hepatitis B Virus (HBV) are transmitted by exposure to contaminated blood or blood and plasma products, exposure to certain body tissues or fluids, by sexual contact or by an infected mother to the newborn child. According to World Health Organization (WHO), there are approximately 38 million people living with HIV/AIDS in the world.
Hepatitis C virus (HCV) and hepatitis B virus (HBV) have affected 71 million and 257 million people worldwide respectively. The screening of donated blood and blood components represent critical processes that should be followed to ensure that blood units are safe. Serological screening assays have greatly reduced, but not eliminated, the risk of transmission of viral infections by transfusion of blood and blood products.
Studies have shown that testing for viral nucleic acids can further reduce the transmission risk of these agents in blood donations.
Features Include
Technical Specs
Workflow Overview for the Atila Blood Screening Assay Kit
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.
Collect plasma sample.

Add 1/10 elution volume of processing control (5uL) to plasma sample.

Mix 12.5 µL extracted sample with 12.5 µL reaction mix in a new tube.
Load mix into PCR plate wells.

Transfer the reaction mix to a PCR machine.