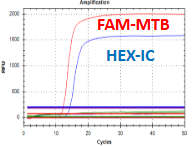
AmpFire MTB Assay
The AmpFire MTB Assay kit is an extraction-free, in vitro nucleic acid isothermal amplification assay with real-time fluorescence detection, intended for the qualitative detection of DNA from Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium africanum, Mycobacterium canettii, and Mycobacterium microti in raw sputum or concentrated sputum sediment prepared from induced or expectorated sputum samples.
Each kit includes 96 tests.
This product is for Research Use Only.
AmpFire MTB Assay
Active pulmonary TB is a highly infectious airborne disease. All patients in healthcare facilities with suspected TB should be maintained in airborne infection isolation (AII) according to recommended infection control guidelines. The testing of either one or two sputum specimens by the AmpFire MTB Assay may serve as an alternative to serial acid-fast stained sputum smears as an aid in the decision of whether continued infection control precautions are warranted in patients with suspected pulmonary tuberculosis.
Sputum specimens that are AFB smear-negative but subsequently TB culture positive have lower MTB-complex organism loads than specimens that are AFB smear-positive. Because of the greater sensitivity of the AmpFire MTB Assay for the detection of MTB-complex than that of acid-fast microscopy, MTB-complex may be detected by the AmpFire MTB Assay in AFB smear negative samples. Patients with HIV infection and pulmonary TB are known to have lower organism loads of MTB-complex in their sputum specimens relative to HIV-uninfected patients, despite more rapid disease progression if untreated. As a consequence, sputum specimens from HIV-infected patients with pulmonary TB tend to be AFB smear-negative more frequently than HIV-uninfected patients. Overall rates of detection of MTB-complex with the AmpFire MTB Assay may be lower in settings with a high percentage of HIV-infected patients because these patients are more likely to produce AFB smear-negative specimens with low organism loads.
Features Include
Technical Specs
Workflow Overview for the AmpFire MTB Assay (raw sputum or sputum sediment samples)
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.

Clinical Sample in Atila Sample Collection Tube

Add Lysis Buffer

Heat at 95 degree C for 20 minutes
Load 10uL processed sample and quickly spin down

Isothermal Amplification for 60 min