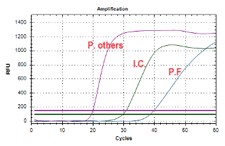
AmpFire Malaria Detection Assay
The AmpFire Malaria Detection Assay kit is an extraction-free, in vitro nucleic acid isothermal amplification assay with real-time fluorescence detection, designed for the qualitative detection of DNA from Plasmodium falciparum, Plasmodium knowlesi, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax. The assay also differentiates Plasmodium falciparum, the most common malaria-causing pathogen, from other Plasmodium species across various human sample types.
MAL-100-RUO (Liquid Format), MAL-96-RUO (Zebra BioDome Format)
This product is RUO (for research only).
AmpFire Malaria Detection Assay
Malaria is a serious and sometimes fatal disease caused by a parasite that commonly infects a certain type of mosquito which feeds on humans. People who get malaria are typically very sick with high fevers, shaking chills, and flu-like illness.
Four kinds of malaria parasites infect humans: Plasmodium falciparum, P. vivax, P. ovale, and P. malariae. In addition, P. knowlesi, a type of malaria that naturally infects macaques in Southeast Asia, also infects humans, causing malaria that is transmitted from animal to human (“zoonotic” malaria). P. falciparum is the type of malaria that is most likely to result in severe infections and if not promptly treated, may lead to death. Although malaria can be a deadly disease, illness and death from malaria can usually be prevented.
About 2,000 cases of malaria are diagnosed in the United States each year. The vast majority of cases in the United States are in travelers and immigrants returning from parts of the world where malaria transmission occurs, including sub-Saharan Africa and South Asia.
Globally, the World Health Organization estimates that in 2020, 241 million clinical cases of malaria occurred, and 627,000 people died of malaria, most of them children in Africa. Because malaria causes so much illness and death, the disease is a great drain on many national economies. Since many countries with malaria are already among the poorer nations, the disease maintains a vicious cycle of disease and poverty.
The Atila AmpFire Malaria Assay, is an isothermal nucleic acid amplification assay for the qualitative detection of Plasmodium falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi. Specific primers and fluorescent probes are used to amplify regions of pathogen genomic DNA under isothermal conditions. The assay detects Plasmodium falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi and allows simultaneous identification of the Plasmodium falciparum.
Features Include
Technical Specs
Workflow Overview for the AmpFire Malaria Detection Assay(MAL-100-RUO)
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.

Clinical serum sample in Atila sample lysis buffer.
Transfer 5µL to plate or strip. Add 20µL Reaction Master Mix.

Add 20µL Reaction Master Mix.