AmpFire HPV High Risk Genotyping
The AmpFire HPV High Risk Genotyping assay is an extraction-free, in vitro nucleic acid isothermal amplification test with real-time fluorescence detection, designed for the qualitative genotyping of high-risk Human Papillomavirus (HPV) types 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68 from different sample types..
Each kit includes 100 tests.
This product is CE-IVD certified. It is for research use only in US.
AmpFire HPV High Risk Genotyping
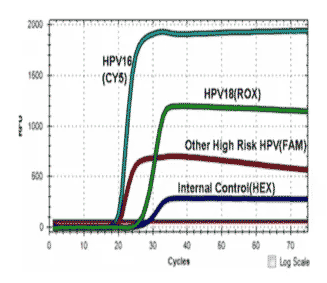
The AmpFire HPV High Risk Genotyping is an isothermal nucleic acid amplification assay for the qualitative genotyping of high risk types of human papillomavirus (HPV). High risk HPV specific primers and fluorescent probes are used to amplify regions of viral genomic DNA including E6/E7 regions under isothermal conditions.
The assay genotypes HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68. Cervical specimens that may be tested with the Atila AmpFire HPV High Risk Genotyping assay include dry cervical swab, PreservCyt Solution (using an aliquot that is removed prior to or after processing for the PreservCyt Pap test), BD SurePath Preservative Fluid (using an aliquot that is removed prior to or after processing for the BD SurePath Pap test and formalin-fixed paraffin-embedded (FFPE) tissue specimens.
Human papillomavirus (HPV) is the most common sexually transmitted virus in the world. In most cases, HPV infections are transient, and the body will clear the virus on its own. In some instances, however, the virus is persistent and will cause normal cells to become abnormal, leading to cancer.
These oncogenic types of HPV are classified as high risk and include HPV 16 and 18. Other types are considered low risk, as they do not appear to cause cancer. According to the World Health Organization (WHO), HPV is the second biggest cause of female cancer mortality worldwide, claiming about 250000 lives annually.
In Europe alone, the disease claims about 15000 lives each year. It is estimated that nearly 70% of cervical cancers are caused by HPV types 16 and 18.
Features Include
Fast Speed
Easy To Use
Flexibility
Low Cost
Technical Specs
Assay Targets
Reaction Time
Sample Types
Sensitivity
Instruments
Workflow Overview for the AmpFire HPV High Risk Genotyping
This section is for demonstrative purposes only and may be incomplete or inaccurate. Always refer to the product instructions for precise guidelines and directions.